Zinc is incorporated into a number of metalloproteins in cells, some of which are enzymes. Within these enzymes the zinc can either function as a catalyst or as a structural component. The cell stores zinc in a protein called metallothionein which acts to control the amount of zinc available to enzymes and also reduces the free zinc ions within the cytosol. Zinc within the cell is in a constant flux between metallothionein and enzymes under the normal process of cellular metabolism. The regulation of free zinc ions within the body by metallothionein is important because if the concentration within cells rises, the free zinc ions can interfere with other metal dependent processes that involve metals such as calcium. To overcome this problem, the cells bind zinc tightly to metallothionein so that the concentration of cellular free zinc ions is always kept low.
Metallothionein is a protein that is made up of 60 to 68 amino acids, with roughly one fifth being cysteine. Metallothionein is able to bond seven molecules of zinc. Within the molecule itself the zinc ions are bound tetrahedrally to four of the cysteine residues, shielding the zinc from the exterior of the protein. Evidence suggests that their exists within cells a metallothionein / thionein couple which acts to homeostatically regulate free zinc ions (figure 1). Increasing amount of free zinc ions within the cell result in the synthesis of the protein thionein within the cells. This thionein binds to the free zinc ions to form metallothionein, thus reducing the cellular levels of free zinc ions. When free zinc ion concentration falls and zinc is needed for the formation of important enzymes, the metallothionein releases its zinc ions resulting in the formation of the protein thionein.
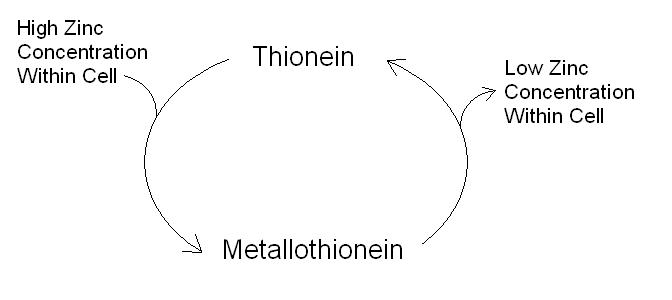
Figure 1. The metallothionein / thionein cycle1.
The association of the zinc with the cysteine residues in the metallothionein is important because cysteine contains sulfur groups which are the key to releasing the zinc from the storage protein. Zinc is stored in the protein bound to the sulfur residues of the cysteine when they are in a reduced state. Oxidising the sulfur residues results in an uncoupling of the zinc ion with its release from the protein. In contrast, reduction of the sulfur residues causes them to bind zinc ions, sequestering them from the rest of the cell. This oxidation reduction system is co-ordinated from the cellular environment (figure 2). A more oxidising state of the cell, results in the release of zinc from its metallothionein binding protein, and a more reducing state results in an increase in binding.
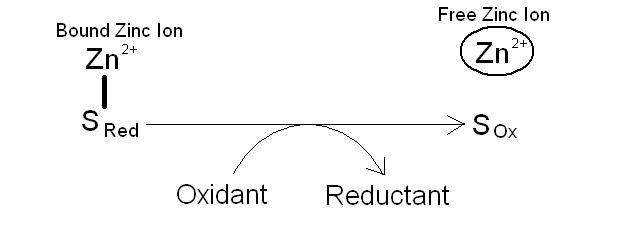 Figure 2. The binding of zinc ions to sulfur in cysteine residues within metallothionein. Oxidation of the sulfur causes the release of the zinc ions1.
Figure 2. The binding of zinc ions to sulfur in cysteine residues within metallothionein. Oxidation of the sulfur causes the release of the zinc ions1.
RdB
