 Antioxidants are important biochemically. They protect cells from oxidative stress through their ability to react with free radicals and this can prevent the propagation of free radical chain reactions. While free radicals play an important role in cellular chemistry, their overproduction can lead to disease. Common dietary antioxidants include vitamin C, vitamin E and a number of phytochemicals including the polyphenols and carotenoids. However, antioxidants can also be synthesised endogenously. In this regard, a number of endogenously synthesised chemicals are known to have antioxidant effects in humans and these include alpha lipoic acid, coenzyme Q10 and glutathione. However, of all the endogenously synthesised antioxidants, uric acid (urate in its dissociated form) is perhaps the most important quantitatively. It is estimated for example that 35 to 65 % of all antioxidant capacity in blood is provided by urate. Urate is a produce of the natural breakdown of nucleic acids, including purine and pyrimidines.
Antioxidants are important biochemically. They protect cells from oxidative stress through their ability to react with free radicals and this can prevent the propagation of free radical chain reactions. While free radicals play an important role in cellular chemistry, their overproduction can lead to disease. Common dietary antioxidants include vitamin C, vitamin E and a number of phytochemicals including the polyphenols and carotenoids. However, antioxidants can also be synthesised endogenously. In this regard, a number of endogenously synthesised chemicals are known to have antioxidant effects in humans and these include alpha lipoic acid, coenzyme Q10 and glutathione. However, of all the endogenously synthesised antioxidants, uric acid (urate in its dissociated form) is perhaps the most important quantitatively. It is estimated for example that 35 to 65 % of all antioxidant capacity in blood is provided by urate. Urate is a produce of the natural breakdown of nucleic acids, including purine and pyrimidines.
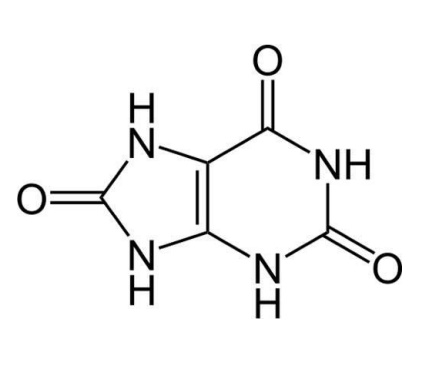
Urate can react with hydroxyl radicals to form a urate radical. However, the ring structure of urate allows the unpaired electrons to be delocalised around its structure and the urate radical will not react with molecular oxygen to form a peroxyl radical. The urate radical is then reduced back to urate through eh action of vitamin C. Urate may also interact with the superoxide radical and singlet oxygen. Some evidence suggests that urate may also interact with ozone and nitrogen dioxide to prevent damage from air pollution. Urate can also chelate metal iron and prevent the nitration of proteins. High levels of urate in the blood suggest that it is quantitatively important in this role and experimental data supports this contention. Ring structures as found in urate are commonly found in antioxidants. Polyphenols are also antioxidants on account of their ability to delocalise the charge from unpaired electron around their structure.
Eat Well, Stay Healthy, Protect Yourself
RdB
