Anthocyanins are a group of flavonoids that are responsible for the blue, purple and red colouration of berries. They are important in human nutrition because they are thought to confer significant health benefits to those who regularly consume them. In particular, anthocyanins are thought to interact with vascular tissue and prevent endothelial dysfunction and therefore reduce the risk of cardiovascular disease. Anthocyanins are extensively metabolised in the tissues of the gut before absorption and following berry rich meals the plasma is made up of both anthocyanin and anthocyanin phase II conjugates. Estimates of the plasma concentrations suggest that a 500 mg anthocyanin intake can raise levels of plasma anthocyanins to roughly 100 nmol/L, with the average consumption of anthocyanins suggested to be in the region of 12 mg per day in the United States. Once in the plasma, anthocyanins and their phase II conjugates are rapidly metabolised to other phenolic substances before elimination but this route of degradation is not fully understood.
Studies have attempted to identify the elimination pathways of flavonoids using radiolabeled molecules. For example, one study1 labelled cyanidin 3-glucoside with isotopes on the A and B ring to trace its metabolism in human subjects. The radiolabelled anthocyanin (500 mg; figure 1) was fed to 8 subjects and the metabolite concentrations analysed from tissues. During the 48 hour period following consumption the amount of radiolabeled carbon detected in urine, breath and faeces was 44 %. The maximal rate of elimination was at 30 min following ingestion and the elimination was greatest between 0 and 1 hour for urine, at 6 hours for breath and between 6 and 24 hours for faeces. The highest concentration of radiolabelled carbon was detected in urine suggesting that this was the main route of excretion. The detection of radiolabelled carbon in the breath over the 48 hour period suggests absorption of low molecular weight faecal metabolites from the colon via fermentation to carbon dioxide.
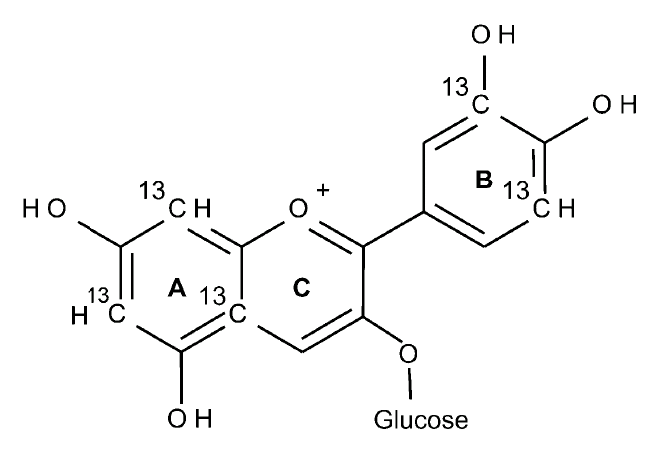
Figure 1. The radiolabelled cyanidin 3-glucoside molecule with radiolabelled carbon on the A and B rings.
The researchers also analysed the metabolites containing the radiolabelled carbon and detected phenolic, hippuric, phenylacetic and phenylpropanoic acid metabolites, but noted that other metabolites likely escaped detection. These metabolites were present in the circulation for 48 hours following ingestions of the original parent molecule. Based on the elimination of the radiolabelled carbon in this study the bioavailability of cyanidin 3-glucoside was estimated to be ~12 %. Previous estimates of anthocyanin bioavailability have been much lower (~0.4 %). However, this research suggests that anthocyanins might be as bioavailable as other flavonoid subclasses such as catechins (flavan-3-ols) and flavones which have bioavailabilities that range from 2.5 to 19 %. As with other metabolite studies looking at phytonutrients, there was considerable variation between the subjects in terms of the recovery of the radiolabeled tracer (ranging from 15 to 99 %) suggesting that other factors, perhaps genetic, influence the metabolism of anthocyanins.
RdB
