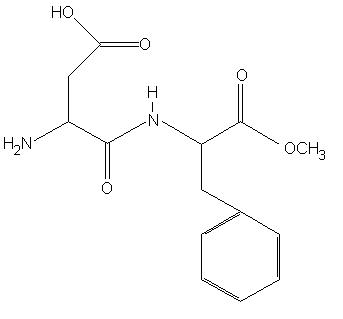
Aspartame (3-amino-N-(α-carboxyphenylethyl) succinamic acid, N-methyl ester), is a methyl ester of the dipeptide aspartyl-phenylalanine (figure 1). Aspartame is used as a sweetening agent in many foods on account of the fact that is roughly 180 times sweeter than table sugar (sucrose). Aspartame contains roughly 4 kilocalories per gram, but is used in such small quantities that its calorific contribution to food is negligible. Aspartame goes under the trade name of NutraSweet, AminoSweet, Equal or Spoonful, and was originally used as a sugar substitute in low sugar products. However, increasingly it is being used as an ingredient in foods where a sweet taste is seen to be advantageous such as fruit squashes, soft drinks and confectionary, in addition to sugar. Aspartame is controversial because it is linked to neurotoxicity in some individuals and as such has attracted much attention.
Figure 1. The molecular structure of aspartame.
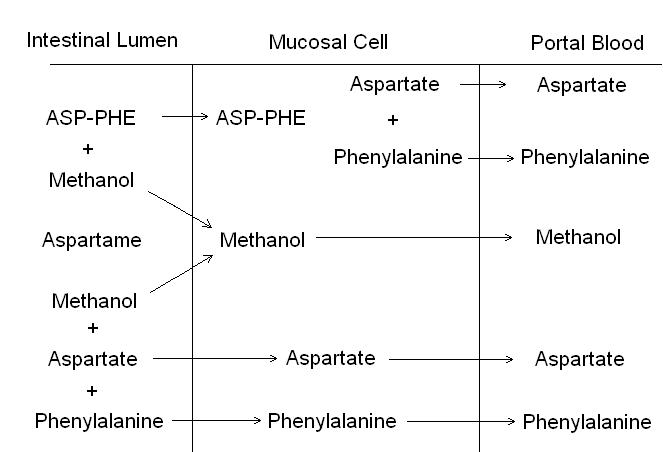
Aspartame is not affected by the acid within the stomach and so digestion begins in the small intestine. Here, aspartame is digested in one of two ways (figure 2). Firstly, aspartame is demethylated with the enzyme chymotrypsin to form the dipeptide aspartyl-phenylalanine and methanol. Then the dipeptide is absorbed into enterocytes where it is digested to phenylalanine and aspartic acid. Alternatively brush border peptidases digest the dipeptide in the lumen of the small intestine and absorb the individual amino acids. Once digested to methanol, phenylalanine and aspartic acid, these constituents are absorbed across the epithelial basolateral surface to the circulation and enter normal metabolic routes. Studies performed on monkeys1 confirm that aspartame is digested to methanol, phenylalanine and aspartic acid in the small intestine, and these constituent parts are then absorbed as they would be if derived from the diet.
Figure 2. The hydrolysis of aspartame in the intestinal lumen and the mucosal cell. ASP = aspartate, PHE = phenylalanine2.
Phenylalanine, aspartic acid and methanol all occur naturally in the diets of humans. Phenylalanine and aspartic acid are both amino acids present in dietary protein. Phenylalanine and aspartic acid are both used for the synthesis of new proteins. Phenylalanine is also metabolised via phenylalanine hydroxylase to the amino acid tyrosine and then subsequently to the catecholamines dopamine, noradrenaline and adrenaline. Aspartic acid donates a nitrogen group to form inosine in purine metabolism and is also an important reactant in the urea cycle. Dietary methanol is present in fresh fruits and vegetables in low amounts. Canning can increase the methanol content considerably. In fruits and vegetables it occurs as the free alcohol or esterified to fatty acids, or alternatively as a product resulting from the hydrolysis of methoxy groups of polysaccharides such as pectin.
RdB