Aspartame is a man made chemical that is used extensively in the food industry as an artificial sweetener. When ingested, humans perceive the aspartame molecule to be around 200 times sweeter than table sugar (sucrose). Aspartame is useful as an artificial sweetener because its calorific value is negligible and so holds some nutritional advantages over sucrose, fructose and high fructose corn syrup. In particular, aspartame is of use to the food industry for the sweetening of low calorie soft drinks, that can be marketed as diet versions of those sweetened by calorific sweeteners such as sucrose. Aspartame is marketed under a number of trade names including NutraSweet, NatraTaste and Canderel. Originally used as an alternative to calorific sweeteners, aspartame is increasingly being used alongside calorific sweeteners in various kinds of drinks. This might suggest that aspartame has other properties known to the food industry.
Aspartame is controversial because a growing body of anecdotal reports are suggesting that aspartame use can cause deleterious effects, many of which are neurological. Despite this, the scientific literature shows general consensus that aspartame is safe in amounts over and above those found in food, and finds no inherent danger from its consumption. The contradiction between the anecdotal reports and the scientific literature is interesting because it suggests that either many tens of thousands of individuals are mistaken, or that the methodology in some scientific studies investigating aspartame is flawed. The sheer number of negative effects associated with aspartame use makes it very difficult to dismiss the anecdotal reports as meaningless. Looking deeper into the matter, the discrepancy between public opinion and scientific observation is possibly due to the fact that aspartame is only problematic is a particular sub-group of the population that are sensitive to its effects.
For example, a study published in the Biological Psychiatry Journal in 19931 assessed the effects of aspartame on a group of individuals, some of which were deliberately recruited because they had unipolar depression. In a cross-over, randomised, double-blind design study, 13 subjects received either 30 mg/kg/day aspartame or a placebo for 7 days, followed by a 3 day washout period and then the alternative treatment for 7 days. The results showed that aspartame produced a significant deleterious effect in the subjects when compared to the placebo, which was a similar tablet containing confectionary sugars. No significant difference was found between the deleterious effects reported by those depressed subjects taking medication and those not taking medication. Common symptoms experienced during the study included headache, nervousness, dizziness, trouble remembering, binge eating, lower back pain, nausea, depression, insomnia and temper, suggesting that neurological problems were common.
This study is interesting because the institutional review board ordered the study stopped at mid-point due to the severity of the reactions in some study participants. Three study participants felt that consumption of aspartame had ‘poisoned’ them, and one of these subjects experienced a retinal haemorrhage for the first time in their lives which required hospital treatment. The amount of aspartame in this study was high, but the amount ingested is easily achievable in a normal Western diet. Around 10 to 12 cans of soft drink is all that would be required to provide the same amount of aspartame. This amount is unlikely, but possible, and lower amount could produce tangible but less severe reactions. Therefore this study differs from many other studies for two main reasons. Firstly, a large dose of aspartame was used. And secondly, the study authors deliberately used subjects with a history of depression.
These results suggest that aspartame may only be deleterious to a sub-group of the population and when the dose is large. This would explain the equivocal results seen in the literature. Aspartame is a methyl ester of the amino acids aspartate and phenylalanine (figure 1). During digestion, aspartame is split into methanol, aspartate and phenylalanine, that are then absorbed into circulation (here). Continually elevated plasma concentrations of phenylalanine are known to cause neurological problems in the genetic disorder phenylketonurea (PKU). Phenylalanine levels in PKU rise to around 110 µmol/dL, but following ingestion of 34 mg of aspartame reach around 11 µmol/dL. However, continual ingestion of aspartame as might occur in consumption of large quantities of soft drinks, could raise levels considerably further. However it is unlikely that dietary aspartame ingestion would raise levels to the 110 µmol/dL seen in PKU.
Lower levels of phenylalanine could cause neurological problems in particularly sensitive individuals. This may be the reason that those patients suffering from unipolar depression were particularly sensitive to the effects of aspartame. Phenylalanine can cause neurological changes because it is converted in brain tissue to the neurotransmitters dopamine and noradrenaline, which may activate excitatory pathways in the brains. In addition, phenylalanine can decrease transport of tryptophan across the blood brain barrier, and this can decrease brain levels of serotonin. Those sensitive to phenylalanine may therefore be at higher risk of detrimental effects of aspartame. Interestingly, the request for a supply of aspartame from the NutraSweet Company was denied in this study, and the aspartame was purchased from a chemical company. If aspartame was really safe, surely a company would be happy to be involved in a neurological study to show the benefits of its product? 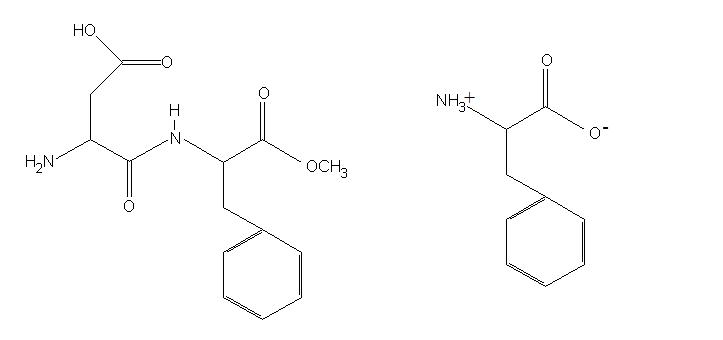 Figure 1. The chemical structure of the artificial sweetener aspartame (left) and the essential amino acid phenylalanine (right).
Figure 1. The chemical structure of the artificial sweetener aspartame (left) and the essential amino acid phenylalanine (right).
RdB
