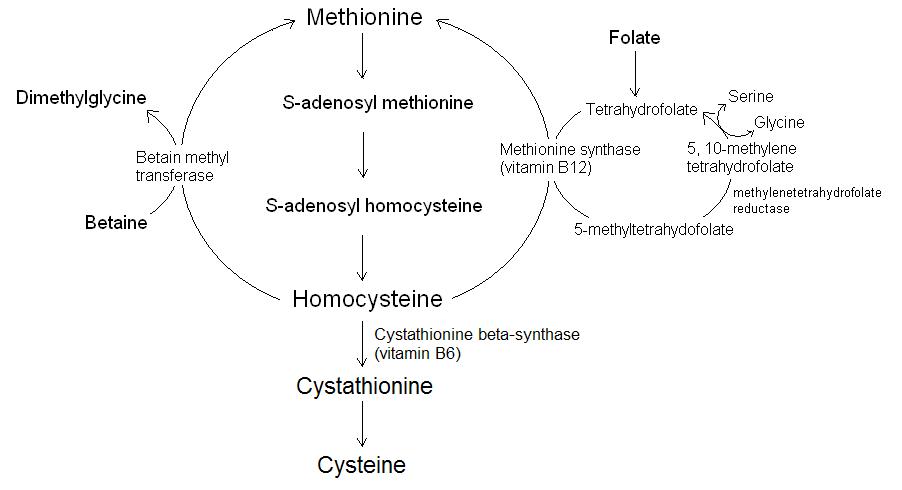
Homocysteine is a non-protein amino acid that is synthesised from the essential amino acid methionine. Excess homocysteine is toxic and has been identified as a risk factor for cardiovascular disease, dementia and neural tube defects during pregnancy. Homocysteinaemia is a genetic conditions whereby a mutation in cystathionine β-synthase (CBS) or methylenetetrahydrofolate (MTHFR) genes causes an elevated level of homocysteine in plasma. In normal healthy people, excess homocysteine forms under conditions of low intakes of folate, vitamin B12 or vitamin B6. Many studies have demonstrated that high homocysteine can be lowered by administration of these vitamins, as well as the non-vitamin substance betaine (trimethylglycine). The only source of homocysteine in the human body is dietary protein containing methionine. After digestion methionine is absorbed and delivered to cells where it is converted to homocysteine. The metabolism of homocysteine is shown in figure 1.
Figure 1. The metabolism of homocysteine.
Homocysteine is therefore formed as a by-product of transmethylation reactions, and under normal conditions most of the homocysteine is converted back to methionine (re‑methylation) or to cysteine (in trans-sulphuration reactions). The homocysteine not removed in this way is converted to the heterocyclic compound homocysteine thiolactone. This synthesis becomes the dominant pathway under conditions where the re-methylation or trans-sulphuration pathways are inhibited (as might occur with vitamin insufficiencies) or through genetic alterations in the CBS and MTHF enzymes. Homocysteine thiolactone is formed by the enzyme methyionyl-tRNA synthetase in all cell types. Homocysteine thiolactone is reactive and causes homocysteinylation through the formation of amide bonds with proteins, particularly the ε-amino groups of protein lysine residues (lysine has two amino groups). The result is damage to components of the body which may include autoimmune disorders, cellular toxicity and atherosclerosis.
RdB