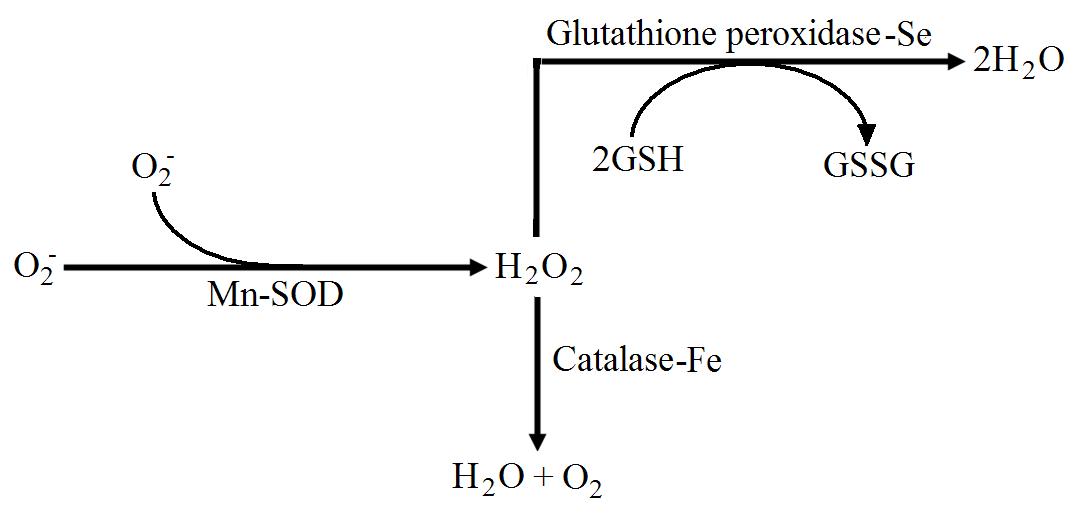
 anganese is required in humans as a co-factor for the enzymes pyruvate carboxylase, arginase, prolidase, glucosyl transferase, phosphoenolpyruvate carboxylase and superoxide dismutase. Superoxide dismutase is of interest nutritionally because it is an enzyme that is important for antioxidant defences and low intakes of manganese are thought to decrease the activity of this enzyme. Superoxide dismutase is present in mitochondria, where it is able to dispose of the superoxide radical (O2–) to create hydrogen peroxide (figure 1). The hydrogen peroxide produced in this reaction is removed from the cell by the action of glutathione peroxidise (here), which requires selenium as a co-factor, or through the action of catalase, which requires iron as a co-factor. Manganese is therefore an important mineral for the antioxidant defences of the cells, and sub-clinical deficiencies may render the cells liable to damage from increases in superoxide concentrations.
anganese is required in humans as a co-factor for the enzymes pyruvate carboxylase, arginase, prolidase, glucosyl transferase, phosphoenolpyruvate carboxylase and superoxide dismutase. Superoxide dismutase is of interest nutritionally because it is an enzyme that is important for antioxidant defences and low intakes of manganese are thought to decrease the activity of this enzyme. Superoxide dismutase is present in mitochondria, where it is able to dispose of the superoxide radical (O2–) to create hydrogen peroxide (figure 1). The hydrogen peroxide produced in this reaction is removed from the cell by the action of glutathione peroxidise (here), which requires selenium as a co-factor, or through the action of catalase, which requires iron as a co-factor. Manganese is therefore an important mineral for the antioxidant defences of the cells, and sub-clinical deficiencies may render the cells liable to damage from increases in superoxide concentrations.
Figure 1. The metabolism of the superoxide radical (O2–) to hydrogen peroxide (H2O2) by manganese dependent superoxide dismutase. The hydrogen peroxide is metabolised to water by either iron dependent catalase or selenium dependent glutathione peroxidise.
Total manganese pools in humans are around 15 mg as either the Mn2+ or Mn3+ ion. Manganese absorption in humans is only around 3 to 4 %, but deficiency is very rare because manganese is widespread in plant foods. However, the effects of low intakes over extended periods is not known. High intakes of calcium, phosphorus, fibre and phytate may inhibit absorption, and regular consumption of these nutrients may require increased manganese intakes. Because manganese is transported bound to transferrin, manganese retention is susceptible to interference from iron (here). Manganese absorption in humans shows great variability between subjects although the reason for this is not clear. In addition, most manganese is excreted in the bile, which makes normal sample methods for estimating retention difficult. Therefore many studies have used radioisotopes to trace manganese through the body in order to elucidate the factors affecting its absorption.
For example, one study1 investigated the whole body retention of manganese using 54Mn and a whole body counter method after ingestion of infant baby formula meal by 14 healthy adults. In the first 10 days of the study, excretion rates of manganese increased dramatically as the supplemental manganese causes a decrease in the manganese retention by the subjects. After this 10 day period, the researchers observed reproducible retention figure in the subjects, but noted large inter subject variation on the amount of manganese retained. The mean retention of manganese was 2.9 % at day 10, but varied between subjects from 0.9 % to as high as 9.2 % of the ingested manganese. This was reflected in large variations in the rate of excretion of radiolabelled manganese between the subjects. In addition, there was a large variation in absorption, that was estimated at between 0.8 and 16 %.
Estimating the absorption and retention of minerals in humans is incredibly difficult because many factors can influence both. However, these results suggest that manganese absorption and retention shows large variation between subjects and this is in broad agreement with other studies investigating the metabolism of manganese. Other studies have shown slightly higher absorption in women compared to men (here), possibly due to differences in iron metabolism. These data also highlight the large biochemical individuality between subjects when it comes to some nutrient factors. Current recommendations are to ingest around 2 to 5 mg/d of manganese, which is widely distributed in nature in whole grains, fruits, nuts and seeds. However, concentrations in plant material depends on the quality of the soil and so care should be taken to eat a high quality diet. The link between manganese and superoxide dismutase further confirms the role of metal ions in antioxidant defences.
RdB