Flavan-3-ols are a group of flavonoids present in commonly eaten foods such as tea, apples and wine. The main flavan-3-ols are (+)-catechin and (-)-epicatechin, which can form various compounds by bonding to gallic acid (figure 1). Epidemiological evidence suggests that high intakes of flavan-3-ols, particularly from green tea, reduce the risk of cardiovascular disease and cancer. The mechanisms of action of flavonoids is not fully understood, and while much attention has been paid to their antioxidant function, evidence suggests they may also function to regulate gene transcription. As well as being present as isolated compounds, flavan-3-ols are also present in dimmers and oligomers called procyanidins (figure 2 and figure 3) which can be found in foods such as cocoa and in supplements made from pine bark. Because procyanidins have a higher molecular weight than single flavan-3-ols, questions remain about their absorption.
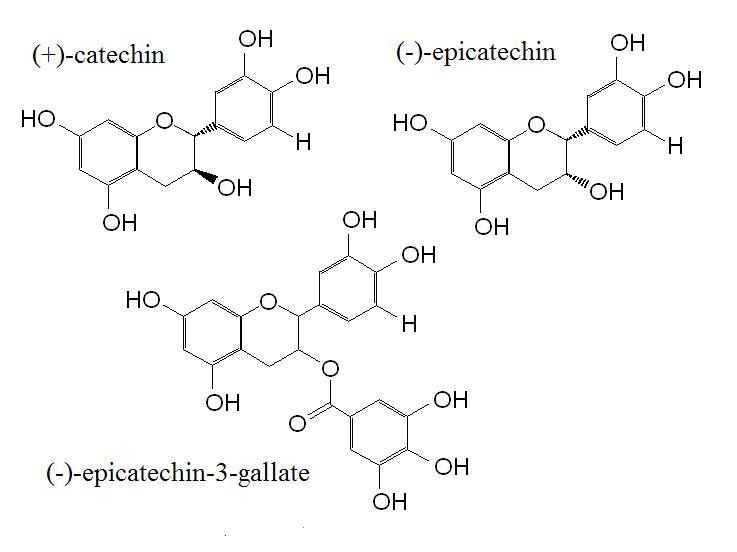 Figure 1. The structure of the flavon-3-ols (+)-catechin, (-)-epicatechin and (-)-epicatechin-3-gallate.
Figure 1. The structure of the flavon-3-ols (+)-catechin, (-)-epicatechin and (-)-epicatechin-3-gallate.
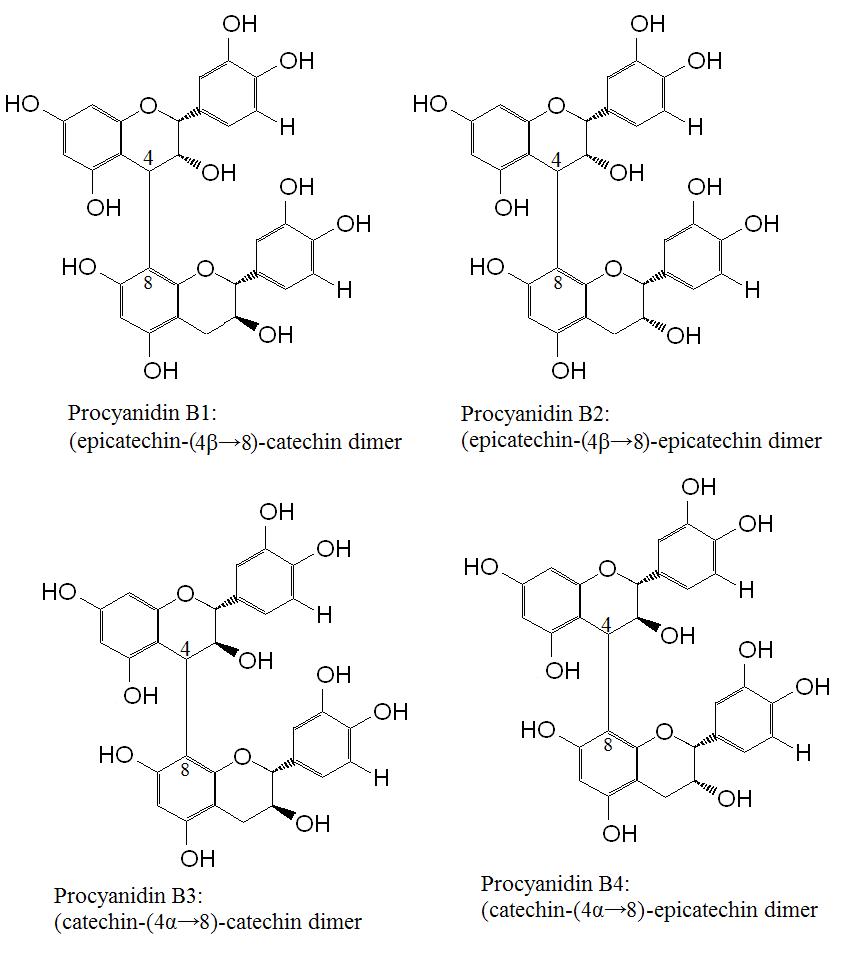 Figure 2. Procyanidins dimers B1, B2, B3 and B4. This series of procyanidins are linked by C4 to C8 bonds. Increasing the chain lengths forms trimer, tetramers and oligomers of varying lengths.
Figure 2. Procyanidins dimers B1, B2, B3 and B4. This series of procyanidins are linked by C4 to C8 bonds. Increasing the chain lengths forms trimer, tetramers and oligomers of varying lengths.
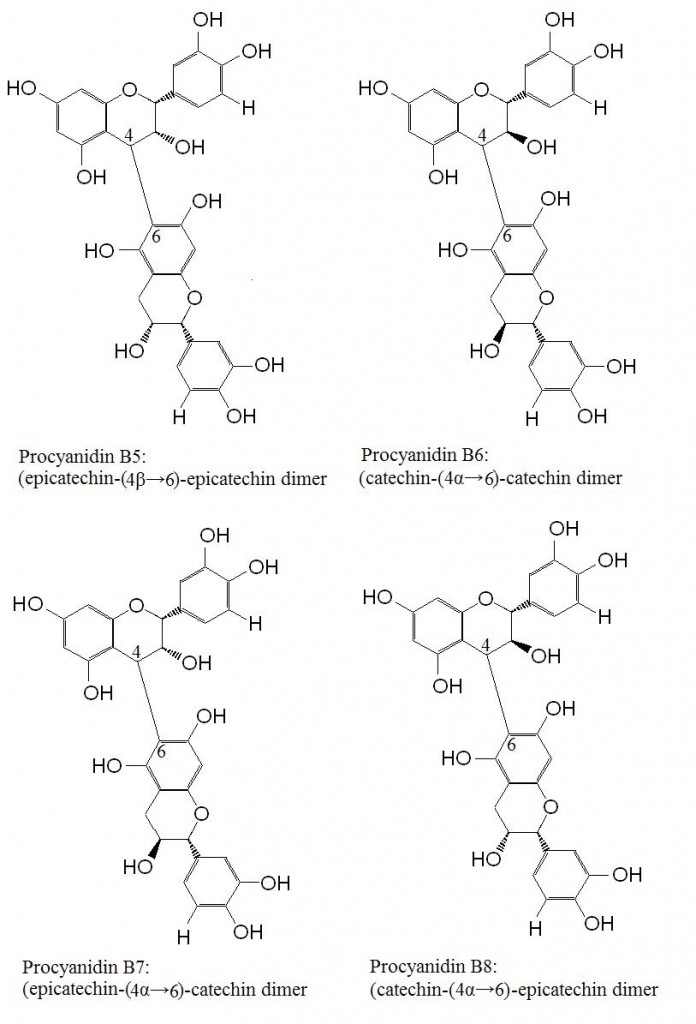 Figure 3. Procyanidins dimers B5, B6, B7 and B8. This series of procyanidins are linked by C4 to C6 bonds. Increasing the chain lengths forms trimer, tetramers and oligomers of varying lengths.
Figure 3. Procyanidins dimers B5, B6, B7 and B8. This series of procyanidins are linked by C4 to C6 bonds. Increasing the chain lengths forms trimer, tetramers and oligomers of varying lengths.
Studies have shown that plasma level of procyanidins reach levels 2 orders of magnitude lower than with flavon-3-ols, suggesting poor bioavailability. In addition, the suggestion that procyanidins can be metabolised to their monomeric flavon-3-ol subunits appears not to be the case. For example, researchers1 have investigated the contribution of procyanidins to the systemic pool of flavon-3-ols by supplementing healthy subjects with test drinks containing flavon-3-ols, procyanidins with a polymerisation ranging from 2 to 10 units, or a combination of the two. Consumption of the combination drink and the flavan-3-ol only drink resulted in similar plasma concentrations of circulating total (methylated and aglycone) flavan-3-ols (880 and 863 nmol/L, in flavan-3-ol only and combined drink, respectively). However, consumption of the procyanidin only drink caused only negligible increases (80 nmol/L) in circulating flanan-3-ol concentrations. This suggests that procyanidins do not contribute significantly to the circulating pool of flavan-3-ols in humans.
In addition, the flavan-3-ol only and combination drink caused significantly more excretion of flavan-3-ols in the urine from 0-24 hours, compared to the procyanidin only drink. This confirms that the flavan-3-ols were not absent from the plasma because they were present in the urine. The combination drink also raised 24-hour urine concentrations of 5-(3,4-dihydroxyphenyl)-γ-valerolactone (γ-VL) to 93 µmol/L, whereas the flavan-3-ol and procyanidin drinks caused increased to 34 and 53 µmol/L, respectively. Macrobiotic metabolism of flavan-3-ols are thought to cause increased production of catabolised ring-fission products such as γ-VL, which can subsequently be absorbed. This evidence suggests that procyanidins contribute significantly to γ-VL absorption and suggest that this might be a possible mechanism by which procyanidins can alter physiological parameters. The finding that γ-VL can be formed from flavan-3-ols and procyanidins also further highlights the importance of microbial gut populations in the digestion and absorption of dietary components.
Detection of procyanidin B5 was not observed in any of the test subjects plasma, but B2 was observed in 10 of the 12 subjects. This indicates that small amounts of some procyanidins are absorbed intact, although plasma concentrations were only 4.0 nmol/L 2 hours after consumption. Treatment of the sample with glucuonidase and sulfatase enzymes did not increase the quantity of B2 recovered suggesting that it had not been O-sulfonated or O-glucuronidated during absorption or first pass metabolism. The detection of B2 in only some subjects suggests biological variability in the absorption and metabolism of flavonoids as has been shown previously. Taken as a whole, these results provide a mechanism by which procyanidins may be absorbed, This suggests they may cause physiological interactions as suggested by epidemiological studies. However, it would appear that this absorption does not involve the conversion of procyanidins to significant levels of monomeric flavan-3-ol units.
RdB
